Droplet microfluidic technology for detecting and screening disease biomarkers
According to the consulting report of Meymers, biomarkers can reflect the changes in cells or molecules related to diseases in the human body. The reliable detection of biomarkers is very important for clinical diagnosis and therapeutic applications. Common disease biomarkers include genes or gene mutations, proteins, and single cells found in disease-associated tissues and biological fluids (eg, blood, urine, saliva, etc.). Unfortunately, for many diseases, the low abundance and low sample volume of human sample biomarkers make standard batch platforms such as 96-well plates ineffective for reliable detection or screening. In addition, most standardized bulk protocols largely fail to detect biomarkers directly from clinical patient samples, and therefore require complex multi-step sample preparation methods, which may be very time consuming and may also be delayed in clinical settings. Patient care. Thus, there is a need in the market for a sensitive, rapid, automated, high-throughput sample-to-answer device platform that can be used to detect and screen disease biomarkers.
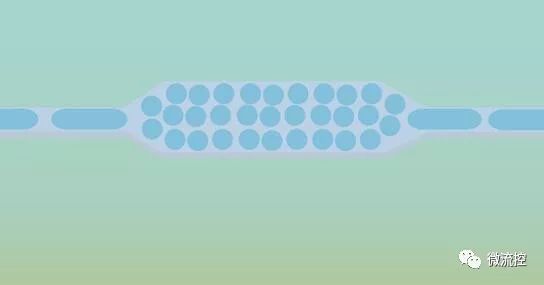
Because microfluidic technology can accurately manipulate small volumes of fluid, it has become an effective tool for biomarker detection. In particular, a large number of samples are digitized by the droplet microfluidics technology into a large number of separated micro-volume fluids (fL-nL), providing a promising solution for high-sensitivity and high-throughput biomarker detection. Achieving a significant reduction in volume by digitizing the biomarkers in the droplets helps determine the same reduction in background, as well as a dramatic increase in the local concentration of biomarker target. This in turn enhances the signal-to-background ratio of each separation reaction, ultimately increasing the overall sensitivity and speed of the assay. In addition, running millions of individual biomarker reactions in parallel from the entire sample not only helps detect rare targets but also enhances the screening of multiple samples. Over the past few decades, researchers have developed a large number of microfluidic droplet platforms to detect, quantify, and screen nucleic acids, proteins, and single cells from human samples to study bacterial infections such as cancer. For example, digitizing pathogenic bacterial single cells from human biological fluids (such as blood) allows researchers to perform rapid diagnostic testing of pathogen identity and drug susceptibility to shorten the turnaround time of clinical microbiological diagnostic workflows. Similarly, commercial droplet platforms (such as Bole's ddPCR) provide a full suite of tools for detecting and quantifying cancer-specific mutations with single-molecule sensitivity in human genetic samples.
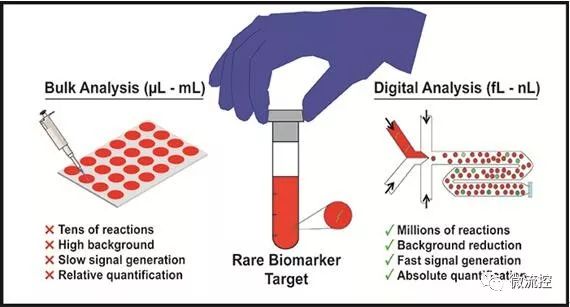
The potential for rapid diagnosis and screening has motivated researchers to become increasingly interested in droplet microfluidics, and it has also been increasingly used to detect novel biomarker targets. However, most droplet platforms rely extensively on fragmented workflows, which require off-chip steps for sample purification and droplet culling. The result is that fully integrated sample-to-answer droplet platforms have not yet achieved clinical Implementation. To achieve this goal, seamlessly integrated sample preparation techniques, and platform automation from patient samples to clinically feasible solutions, remain key components that will provide a platform based on droplet microfluidics for the clinical environment in the future.
UV Film,UV Screen Protector,UV Curing Screen Protector
Shenzhen Jianjiantong Technology Co., Ltd. , https://www.jjthydrogelprotector.com