Analysis of wireless interface of medical equipment
With the automation of patient monitoring and the surge in demand for data reading accuracy, wireless data transmission is set to become a key medical technology. Industry standards designed to ensure the safe storage and transmission of patient and patient data readings will help doctors handle the growing volume of data in wirelessly connected medical devices.
Based on the above points, this article will introduce three wireless interfaces that can be applied to networked medical devices: Bluetooth, Wi-Fi and ZigBee, and explore different levels of security and interoperability issues.
Application results on networked medical devices such as blood glucose monitors (BGMs) and insulin pumps show that wireless data transmission technology has significant advantages in the medical field for simplifying data collection, simplifying patient monitoring, and simplifying device control. With more convenience and precision, wireless devices are playing an increasingly important role in the 21st century medical field. However, this technology also brings security issues.
Can doctors and researchers trust wireless devices? Given the advantages of wireless data transmission, can doctors and researchers not rely on wireless devices? Or, the following questions are more conducive to understanding the technology:
Which wireless technologies are suitable for medical devices
What is the role of these technologies?
Is there a need for wireless security measures for wireless technologies used in medical device general-purpose procedures?
How industry standards in data collection can make wireless devices play a bigger role
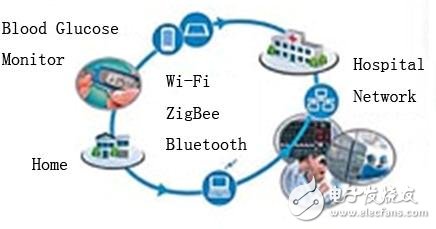
Wireless medical devices can be used at the patient's home, in hospitals, and in assisted living environments.
Bluetooth, Wi-Fi and ZigBee devices (see Figure 1) are available on the market, so open standard wireless technology has become the first choice for medical devices. All open, standardized wireless technologies use a range of international, industrial, and medical frequency bands (ISM bands) that are commonly used in unlicensed medical devices, and comply with industry standards and industry association requirements to ensure interoperability of devices from different manufacturers. Sex.
Microcontrollers are at the heart of medical devices, and their common peripherals include timers, digital-to-analog converters, and USB interfaces. By integrating almost all of the functionality required for medical devices such as blood glucose monitors, microcontrollers provide a reliable, low-cost hardware platform that allows medical device manufacturers to focus on software development.
To meet the relevant safety requirements of the US Food and Drug Administration, many designers separate the transmission function of the device from the primary medical function. For example, even in the event of a failure of the wireless transceiver, the blood glucose monitor (BGM) must continue to record the readings of the instrument.
However, the new generation of wearable devices is subject to strict space constraints and the number of components must be minimized. These devices must use a single microcontroller.
2.1 Home Theater Speaker,2.1 Speaker System Home Theater,Surround Sound Speakers,2.1 Home Theater
GUANGZHOU SOWANGNY ELECTRONIC CO.,LTD , https://www.jerry-power.com